


08 December, 2020

Molecular pores for thought from KAUST Discovery on Vimeo.
Wafer-thin membranes tailored to separate specific molecules from liquids could improve the efficiency of oil refining and pharmaceutical manufacture.Two teams of KAUST researchers have created an ultrathin porous membrane using carefully crafted molecular building blocks, known as trianglamines. “You can imagine it like LEGO,” explains Suzana Nunes, professor of Chemical and Environmental Science and Engineering, “where you take preformed hollow triangles and assemble them together in a flat film.” By first defining the pore size and electric charge of these triangular molecules, they went on to create a membrane that could separate molecules of different sizes and shapes.
Membrane thickness is also critical to filtering efficiency. “For faster filtration, the film needs to be as thin as possible to avoid unnecessary resistance to the solvent passing through,” says Nunes. To achieve this, they separated the two main ingredients (terephthaloyl chloride and the preformed trianglamines) into two different fluids that do not mix (oil and water, respectively), forcing the reaction to occur only at the interface where the fluids met. “We found this formed an extremely thin layer of a few nanometres,” says Tiefan Huang, the lead author, “much thinner than common commercial membranes prepared in this way.” Each film was between 3.5 and 10 nanometres, depending on how long the reaction continued.
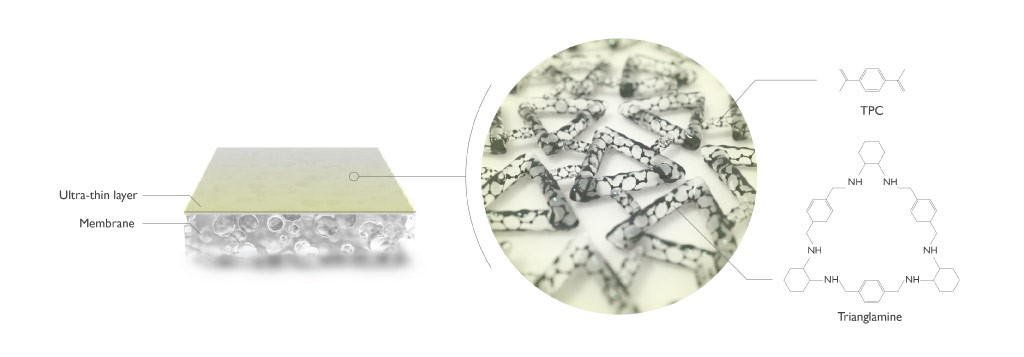
The membranes developed by KAUST scientists did not deteriorate after 48 hours continuous use, withstood exposure to harsh substances and outperformed other membranes that they tested.
© 2020 KAUST; Ivan Gromicho
The team tested their membranes on colored dyes with similar yet distinct molecular sizes. All of their membranes filtered out at least 90 percent of the color molecules that weighed more than 450 grams per mole, far outperforming some of the other membranes they tested. “The membranes’ performance didn’t deteriorate after 48 hours of continuous filtration,” adds Huang. And they even withstood exposure to harsher substances, including acetone and methanol.

Top row: The membrane is cut to size, placed in the filtration apparatus and tested under different applied pressures. Bottom row: The trianglamine aqueous and terephthaloyl chloride hexane solutions are poured onto an ultrafiltration membrane to prepare the ultrathin trianglamine layer.
© 2020 KAUST; Anastasia Serin
Originally published in